Richard E. Chaisson, Johns Hopkins University
In 1993, the World Health Organisation (WHO) declared tuberculosis (TB) a global public health emergency. It urged nations to coordinate efforts to avert millions of deaths.
In January 2020, the WHO declared COVID-19, another airborne infectious disease, a public health emergency of international concern.
The similarity between the global responses to these two pandemics ends there.
The scientific, public health, medical, and pharmaceutical communities’ responses to COVID-19 in the past two years has been spectacular.
Within two weeks of declaring COVID-19 a global emergency, the WHO had convened a meeting of experts and issued a research roadmap. National governments rapidly committed vast sums of money into research at all levels, from basic virology and immunology to clinical care and prevention. Pharmaceutical companies launched development programmes for new products to diagnose, treat and prevent COVID-19.
As a result, diagnostics, therapeutics and vaccines were developed at a dizzying pace, delivering an array of tools to control and end the SARS-CoV-2 pandemic.
The effective and equitable deployment of those tools is a challenge. But no one can say that science has been found wanting in responding to the global crisis.
TB, on the other hand, has not been treated as a true emergency. Yet its worldwide distribution, impact on health, and mortality burden was just as dire. TB incidence remains plateaued at 10 million cases per year. In 2020 case detection fell by almost 20% and mortality rose for the first time in a decade to 1.5 million deaths. These setbacks are directly attributable to the COVID-19 pandemic.
Comparing and learning from responses
The US National Academies of Science, Engineering, and Medicine held a TB workshop in September 2021 to address these challenges and discuss lessons learned from the response to SARS-CoV-2. The workshop highlighted what happens when one epidemic is treated as an emergency and the other is not.
With SARS-CoV-2, the development of diagnostic tests proceeded at speed. Researchers working with government-funded consortia and industry in multiple countries quickly launched treatment trials. It took only weeks to get from protocol development, regulatory review and institutional review board approvals to the launch of phase 3 drug trials. The development of vaccines for COVID-19 went from genetic sequence to phase 1 trials in less than 2 months, phase 3 in another 4 months. Food and Drug Administration approval under an Emergency Use Authorisation came within 11 months. Across the board, institutions treated COVID-19 as an emergency and operated in crisis mode.
Compared with current research on vaccines for TB, the difference is staggering (Table 1).
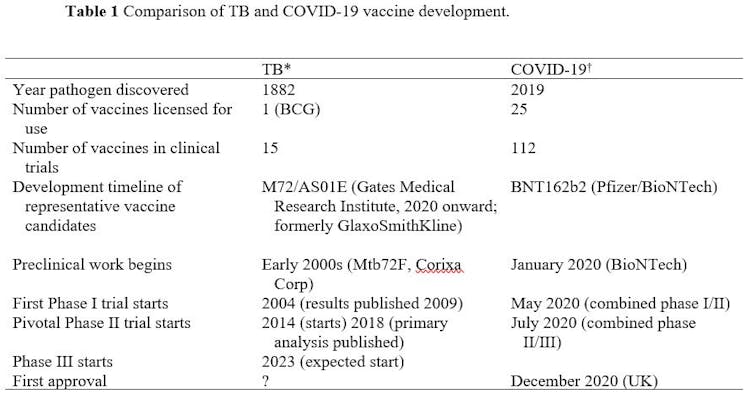
The COVID-19 pandemic is different from the TB pandemic in many ways, with its sudden appearance, rapid worldwide spread, and broad impact on individuals and communities. Nevertheless, TB remains a major killer and the pace of TB clinical research can best be described as glacial.
A comparison of investments in TB research versus research into COVID-19 is astonishing (Table 2).
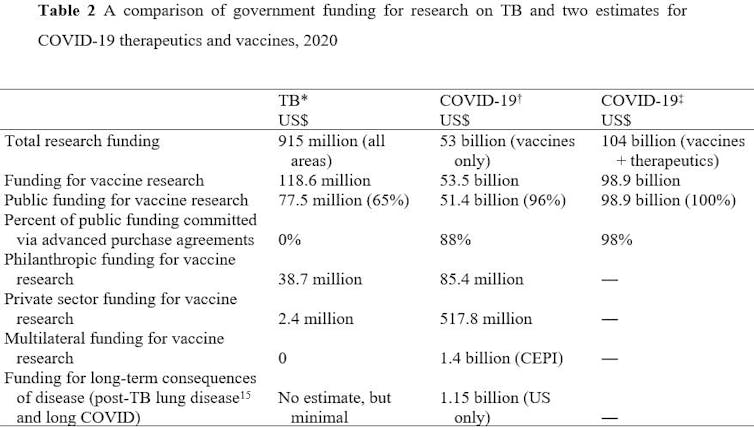
Triumphs despite long timelines
Even with limited funding, there have been some triumphs in TB research in the past decade:
- molecular assays make diagnosis possible in less than 2 hours
- treatment of multidrug resistant TB has been shortened and made easier
- treatment of drug-susceptible TB has been shortened
- treatment of TB infection has been cut, with safer and better tolerated regimens.
But all of these transformative advances took far longer than they should have. Funding opportunities for TB biomedical research are fewer and the reviews of TB applications are slow. The overall timeline for conducting critically important TB research is scandalously long. Most studies are unnecessarily prolonged by long administrative and regulatory review processes.
The broader problem, however, is much larger than the mechanics of individual funding agencies or regulatory bodies.
First, nobody is treating TB as an actual emergency. As we have seen with COVID-19, when everyone thinks it is an emergency, people act differently, and things move rapidly.
Second, the clinical and public health research infrastructure is vastly underfunded and under-supported. COVID-19 has demonstrated what is possible when researchers, funders, and regulatory agencies unite to confront a crisis. Game-changing trials can be conducted in record time without cutting corners and compromising participant safety and scientific integrity, if everyone behaves like it is an emergency. But to do so requires a radical change in mindset in addition to substantially greater human and financial resources.
How to accelerate progress
Operating in crisis mode for COVID-19, TB, or any other health catastrophe, is difficult to sustain. But the COVID-19 pandemic has shown what works to accelerate progress against a global threat.
First, substantial funding for priority research multiplies innovation and progress. As a starting point, governments, pharma/biotech companies, and foundations must increase investment in TB research, at least to the levels laid out in the UN High Level Meeting Report, and make TB a central element in global pandemic response strategies.
Moving forward, the level of ambition must be raised. There is a growing recognition from the COVID-19 experience that the funding targets for TB research are far too low – and the scale-up of newly developed tools is far too slow. Governments and other funders must commit more to end TB by 2030.
Second, the funding timeline can be greatly reduced. If the rationale for faster review of biomedical research in HIV and COVID-19 was that these infections would rapidly spread and kill, then TB grants should likewise be reviewed rapidly.
Third, the regulatory bottleneck must be cleared. There must be more investment in the regulatory and ethics infrastructure (including training and international coordination) so that these vital requirements do not suffocate innovative research.
Finally, governments must treat TB as a central element in global pandemic response strategies. The new focus on pandemic preparedness – most notably the beginning of negotiations at WHO to create a legally binding pandemic treaty or similar mechanism – must include a commitment to end ongoing pandemics such as TB. If an annual 1.5 million deaths due to TB is not a pandemic, then what is?
Advances in TB diagnostics, treatments and prevention need to be pursued and scaled up with the urgency they deserve. If we do not behave like TB is a global health emergency, we will continue to experience unacceptable suffering from a disease that has killed more than 20 million people in this century alone.
The original version of this article co-authored by Mike Frick and Pramay Nahid was first published in The International Journal of Tuberculosis and Lung Disease.
Richard E. Chaisson, Director, Center for Tuberculosis Research, Johns Hopkins University
This article is republished from The Conversation under a Creative Commons license. Read the original article.


